Author: Ginger Ding
China’s pharmaceutical industry continues to grow. It is now the second largest in the world, behind the US. As the pharmaceutical industry develops, it is making large improvements. At the same time, China is working to create a good intellectual property environment, with policies to effectively protect IP. China has formed an intellectual property protection system in which judicial protection and administrative protection are coordinated, and multiple dispute resolution mechanisms are in place to encourage innovation and ensure the viability and continuous development of the innovative drug ecosystem.
Achievements in China’s intellectual property rights
China’s ongoing efforts to protect intellectual property rights and cultivate a strong business environment have gradually been recognized by the international community.
According to the World Intellectual Property Organization’s (WIPO) “2019 Global Innovation Index”, China ranked 14th worldwide in IP protection, up 3 places from 2018.
The “Global Business Environment Report 2020” released by the World Bank in October 2019 shows that China’s series of measures to cultivate a strong business environment have achieved positive results. China’s business ranking jumped from 46th worldwide to 31st in 2018. It has become one of the 10 economies that has made the greatest progress in improving its business environment. These changes in global standing largely come from the ongoing strengthening of IP protections.
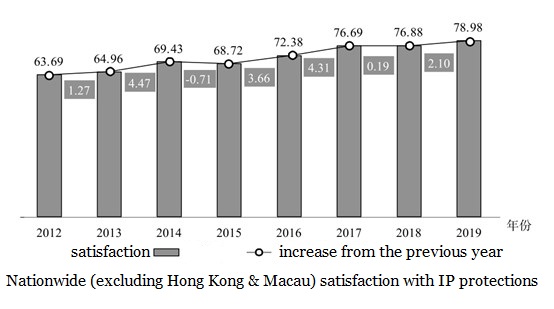
According to a survey, social satisfaction of intellectual property protection increased from 63.69 points in 2012 to 78.98 points in 2019.
According to the 2019 Business Confidence Survey report released by the European Union Chamber of Commerce in China in May, about 60% of the 585 EU companies in China interviewed believe that China’s administrative and judicial protection of intellectual property rights is significantly stronger. The International Intellectual Property Index report released by the American Chamber of Commerce in March pointed out that China has made significant achievements in improving both its online sales environment and drug patent enforcement.

“China’s Intellectual Property Protection and Business Environment New Progress Report (2019)” shows the following:
First, there are plans to promote progress.
- “Opinions on Strengthening the Protection of Intellectual Property Rights”, using legal, administrative, economic, technological, and social governance methods to strengthen IP protection.
- Laws such as “Trademark Law”, the “Anti-Unfair Competition Law” and the “Drug Administration Law” were revised to further increase the penalties for infringements. Meanwhile, the “Foreign Investment Law” and “Regulations on Optimizing the Business Environment” have been newly declared and implemented.
- The 31 provinces in China (including autonomous regions, municipalities) have leading groups to combat IP infringement and counterfeiting which have all been adjusted to have better collaboration.
Second, there is new progress in intellectual property protection.
- The court system is promoting reform of how intellectual property trials are run, as well as strengthening litigation supervision. This results in a more efficient and targeted judicial protection.
- There are continuing efforts to continue to promote collaborative IP rights protection in order to resolve infringement disputes in a timely manner.
- International organizations have carried out special operations in China to crack down on online and cross-border infringement and counterfeiting crimes, with positive results achieved.
Third, the business environment is being strengthened.
- Investment and trade are becoming more convenient in China. Plans are in place to establish six new trade zones, to hold large-scale fairs and expositions, and to revise or abolish more than 400 regulations and related regulations that are inconsistent with the Foreign Investment Law.
- Market access is opening while reducing the range of industrial product production licenses.
- Government services are more optimized and business registration procedures are simplified. They’re improve the efficiency of intellectual property review, shortening the review period, and reducing intellectual property administrative fees.
- The Chinese government is improving the regulatory system, promoting credit supervision, exploring smart supervision, and creating market environment of fair competition and honest operation.
Focus from the highest levels
Chinese state leaders have repeatedly emphasized the importance of strengthening intellectual property protection, severely cracking down on intellectual property infringements in accordance with the law, and creating a world-class business environment.
On April 10, 2018, at his keynote speech at the Bo’ao Forum for Asia 2018 Annual Conference, President Xi Jinping said: “Strengthen intellectual property protection. This is the most important way to improve the property rights protection system and the greatest incentive to improve China’s economic competitiveness. We will improve and strengthen enforcement of the law, significantly increase the cost of violations, and give full play to the role of legal deterrence. We encourage Chinese and foreign companies to carry out normal technical exchanges and cooperation to protect the legal intellectual property rights of foreign-funded companies in China. At the same time, we hope that foreign governments will strengthen the protection of Chinese intellectual property rights.”
Intellectual property protection measures
First, promote the improvement of laws and regulations related to intellectual property rights.
In November 2019, the newly revised Trademark Law revised when harmed companies would receive compensation for multiple malicious infringements of trademark exclusive rights enacted against them. Before, compensation would occur when the infringements were 5 or fewer. It was changed to 3 or fewer. The Trademark Law also introduced several regulations governing trademark registration applications, regulating trademark hoarding and registration. At present, the draft amendment to the Patent Law is being deliberated for the first time by the Standing Committee of the National People’s Congress. The draft amendment clearly establishes a punitive compensation system for infringement, which greatly increases the cost of infringement.
Second, strengthen the protection of the source of intellectual property rights and improve the quality and efficiency of trademark and patent processing.
In 2019, the average processing time for trademark registrations was shortened to 4.5 months while the processing time for high-value patents was reduced to 17.3 months. At the same time, the government cracked down on irregular patent applications and malicious trademark registrations. In 2019, 38,000 irregular patent applications were identified, and 39,000 irregular trademark applications were rejected.
Third, strengthen intellectual property enforcement and enforcement guidance by formulating and improving standards for appraisal and judgment of trademark and patent infringement and issuing guidelines for settling patent infringement disputes.
In 2019, the national intellectual property system handled 39,000 administrative rulings on patent infringement disputes, an increase of 13.7% from the previous year.
Fourth, promote the construction of a rapid collaborative protection mechanism for intellectual property rights.
31 intellectual property protection centers and 20 rapid rights protection centers have been established across China to provide market players with convenient, efficient, and low-cost IP rights protection channels.
The healthcare field
In January 2018, the State Intellectual Property Office issued the “Notice on Printing and Distributing the Catalogue of Key Intellectual Property Support Industries (2018)”. The “List of Registered Drugs in China” clarified China’s key developments areas that urgently need IP support, including 10 industries and 62 subdivided fields. New drug development projects in the healthcare industry are among them.

▲ Notice on Issuing the “Catalogue of Key Intellectual Property Support Industries (2018)” (linked website in Chinese)
On April 3, 2018, a policy from the General Office of the State Council, “Opinions on Reforming and Improving the Supply Guarantee and Use Policy of Generic Drugs” (hereinafter referred to as the “Opinions”), embodied the principle of balancing innovation and imitation. It is a reference document for intellectual property policy innovation in the pharmaceutical field.
At an executive meeting of the State Council in April 2018, they decided to set up a data protection period of up to 6 years for innovative chemical drugs, and to provide compensation for up to 5 years of patent protection period for innovative drugs that have simultaneously applied for listing in China and abroad. The same month, the State Food and Drug Administration released a draft of implementation measures for drug trial data protection.
At present, the fourth revision of the “Patent Law” is in progress. The drug intellectual property protection process is expected to undergo further adjustments.
From April 20 to 26, 2020, 20 departments including the State Intellectual Property Office, the State Administration of Market Supervision, and the Central Propaganda Department jointly launched the 2020 National Intellectual Property Publicity Week with the theme “Intellectual Property and Healthy China”. This has been the 12th consecutive year that the country has held China Intellectual Property Publicity Week, which shows the importance of IP protection.
On April 20, the State Intellectual Property Office launched a plan to implement the “Opinions on Strengthening Intellectual Property Protection” from 2020 to 2021.
The plan includes formulating and revising intellectual property laws and regulations and normative documents, strengthening administrative enforcement and judicial protection of intellectual property rights, expanding foreign collaboration in intellectual property protection, and strengthening intellectual property protection resources:
Article 1. Promote the review of the revision of the Patent Law, introduce a punitive compensation system for infringement, promote the extension of patent validity, and strengthen the protection of pharmaceutical patents. Effectively review the patent process guidelines.
Article 8. Establish an early settlement mechanism for drug patent disputes. (Completed before the end of October 2020)
Article 13. Promote the declaration of regulations on the protection of knowledge around traditional Chinese medicine. (Continuing to advance)
Article 41. Establish a system to publicly release data related to the enforcement of counterfeit drugs on an annual basis. (Completed before the end of July 2020)
Article 50. Carry out enforcement actions against counterfeit medicines, focusing on counterfeit medicines and biological products and other related products. Strengthen international collaboration and exchange corresponding enforcement information with relevant countries. (Continuing to advance)
Article 108. Promote the resolution of issues such as the construction and staffing of the food, drug, police, and intellectual property crime teams. Instruct relevant public security universities to add courses on combating intellectual property rights infringements and increase law enforcement training. (Continuing to advance)

